Author: Atiqul Islam Ahad
Monitoring the rate at which blood is delivered to tissue, medically called perfusion, is crucial in maintaining the health of vital organs. Insufficient perfusion (ischemia) or excessive perfusion (hyperemia), for example, can lead to cellular dysfunction, edema, and ultimately tissue infarction, causing death or permanent disability, among other problems. Over the past few decades, laser speckle imaging (LSI) has replaced some of the conventional perfusion monitoring methods like laser Doppler, ultrasound Doppler, functional magnetic resonance imaging, etc., due to its ability to obtain full field images with high spatial and temporal resolution.

Figure 2.1. Schematic Representation of the Experimental Setup
An 808 nm NIR laser diode (LD808-SEV500; Thorlabs, USA) with an output power of P = 30 mW was used for oblique illumination (~ 45deg) of the sample. The laser was mounted on a thermoelectrically cooled mount (LDM90; Thorlabs, USA) powered by an integrated temperature and current controller (ITC133; Thorlabs, USA). Diverging light from the laser diode was passed through a ground glass diffuser (DG10-220; Thorlabs, USA) and a linear polarizer (LPNIRE100-B; Thorlabs, USA) to illuminate the sample homogeneously. Images were captured continuously while maintaining a constant laser power at different exposure times.
Xavier™ NX (XNX) development kit (NVIDIA, USA) was used in combination with a Raspberry Pi™ HQ camera 12.3 MP (IR filter removed) and a 6 mm CS lens (f/#1.4) kit (B0240; Arducam, USA) for developing a prototype for a touchscreen display with a custom user interface (UI).
A 3D-printed scaffold, as shown in Fig. 3, was used to hold two glass microcapillaries (ID=0.8mm) spaced 5 mm apart and filled with a PDMS solution (base and curing agent mixed in a 10:1 ratio) homogenized with 0.25% w/w titanium dioxide (TiO2) powder (~ 44 μm particle size) as a background scattering medium. PDMS has been increasingly used in characterizing various optical biomedical devices due to its unique optical, thermal, and physical properties that can mimic biological tissues.
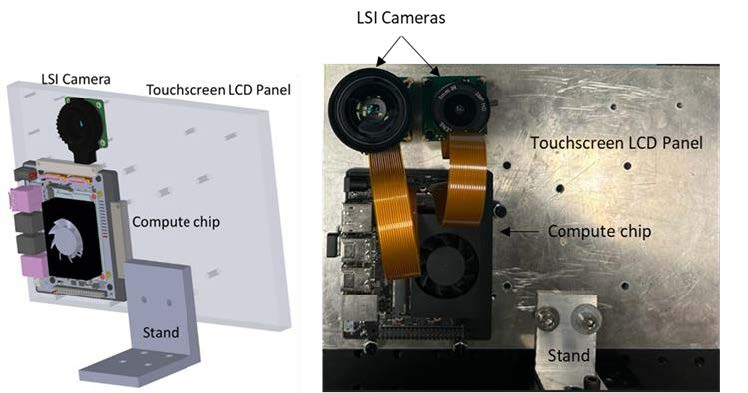
Figure 2.2 Depicts the layout of the prototype and the actual setup, with a NIR camera (CSI camera with IR filter removed) and a computer chip (Xavier NX)
Milestones:
Commercially available LSI-based perfusion monitoring systems are expensive, bulky, and require a desktop arrangement. Some portable LSI devices have also been developed using smartphones and digital signal processors but lack the ability to quantify flow rates due to single exposure settings.
In this study, we aim to develop an LSI system using NVIDIA’s Jetson Xavier NX, a commercially available System on Modules (SOMs) embedded vision processing platform to estimate flow rates in PDMS-based tissue-mimicking phantoms at variable exposures. The Xavier NX can use cheap Raspberry Pi HQ cameras with higher sensitivities to capture images at lower exposure times (< 1 ms) to avoid motion artifacts. Our proposed system captures the laser speckle images of flow phantoms with a static scattering in the background at a video rate of 25 ±2 fps for spatial processing and 5±2 fps for temporal processing. In the presence of static scattering, flow regimes of 2-6 mm/s (slow), 6-12 mm/s (medium), and 12-20 mm/s (fast) were determined, with a high statistical significance. This type of quick semi-quantitative assessment of flow rates can lead to a robust low-latency data processing platform that can be used in real-time. Furthermore, this small SOM system reduces bulkiness as the entire setup could be commercialized into tablet format, with improved portability for point-of-care applications.